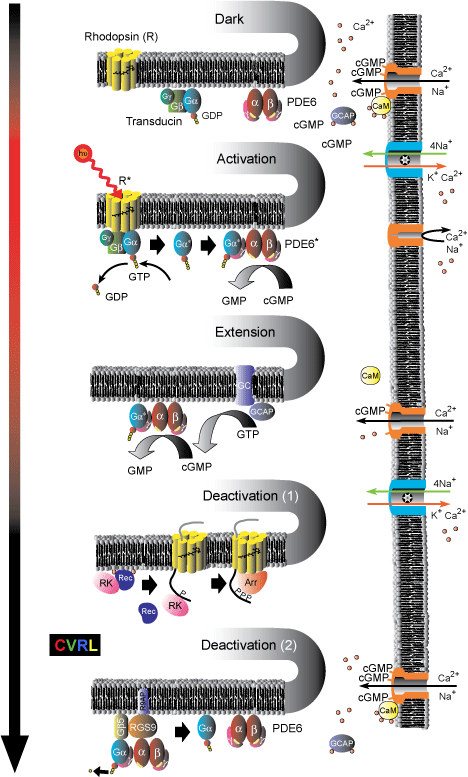

Drawn by Andrew Stockman. Parts of the figure are reproduced in Figure 1 of Stockman, Smithson, Michaelides, Moore, Webster, & Sharpe (2007), and in Figure 1 of Stockman, Smithson, Webster, Holder, Rana, Ripamonti, & Sharpe, (2008).
Processes involved in the G-protein visual transduction cascade.
Dark: The chromophore molecule, 11-cis-retinal, lies in the pocket formed by the seven trans-membrane helices of the G-protein-coupled-receptor-protein rhodopsin (R), to which it is bound covalently via a Schiff-base linkage. Both the heterotrimeric G-protein transducin (Gα-GDP-Gβ-Gγ) and the tetrameric effector enzyme phosphodiesterase (PDE6) are in their inactive states; and the intracellular concentration of cyclic GMP (cGMP) is relatively high. cGMP is thus able to bind to and open cyclic-nucleotide-gated (CNG) channels in the plasma membrane, through which Ca2+ and Na+ ions flow into the cell. The resulting higher Ca2+ concentrations allow Ca2+ ions to bind to calmodulin (CaM), which in its Calcium-bound-form decreases the sensitivity of CNG channels to cGMP; and to bind to guanylate cyclase activating proteins (GCAPs), which deactivates them.
Activation: The absorption of a photon isomerizes the chromophore to its all-trans form, and triggers a conformational change of the rhodopsin into its activated state (R*). R* then activates transducin by catalyzing the exchange of GDP for GTP, which causes the separation of activated α-transducin (Gα*) from the trimer. Gα* in turn activates the phosphodiesterase enzyme (PDE6*) by exposing a site that catalyzes the hydrolysis of cGMP into GMP. The decreased cGMP concentration results in the loss of cGMP from the cyclic-nucleotide-gated (CNG) channels, which then close, blocking the inward flow of Na+ and Ca2+ ions, reducing the circulating electrical current, and hyperpolarizing the membrane voltage. The ionic exchanger continues to pump Ca2+ out of the cell; thereby reducing the cytoplasmic Ca2+ concentration. This decrease triggers the three [Ca2+] sensitive mechanisms of light adaptation described below.
Extension: Two [Ca2+] sensitive mechanism extend the visual response in light. First, cGMP is restored by guanylate cyclase (GC), the activity of which is enhanced by GCAPs. Since GCAPs are inactivated by bound Ca2+ ions (top), their ability to enhance GC increases as the [Ca2+] falls. Second, the sensitivity of CNG channels to cGMP is increased when CaM dissociates from the channels as its Ca2+ ions are lost.
Deactivation (1): R* is deactivated in two steps. First, it is phosphorylated by Rhodopsin Kinase (RK), and then quenched by Arrestin (Arr). The effect of RK is also [Ca2+] sensitive. In the dark (top), RK forms a complex with Recoverin (Rec) and Ca2+ ions, which blocks its activity. As the light level increases and [Ca2+] falls, the complex breaks up, releasing RK.
Deactivation (2): Both Gα*-PDE6* are simultaneously deactivated by the hydrolysis of the attached GTP attached to GDP. This GTPase activity, which is an inherent property of Gα* itself, is substantially enhanced by the GTPase-activating proteins (GAP) RGs9-Gβ5, which are attached to the R9AP membrane anchor protein.
Arshavsky, V. Y., Lamb, T. D., & Pugh, E. N., Jr. (2002). G proteins and phototransduction. Annual Review of Physiology, 64, 153-187.
Burns, M. E., & Arshavsky, V. Y. (2005). Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron, 48, 387-401.
Burns, M. E., & Baylor, D. A. (2001). Activation, deactivation and adaptation in vertebrate photoreceptor cells. Annual Review of Neuroscience, 24, 779-805.
Fain, G. L., Matthews, H. R., Cornwall, M. C., & Koutalos, Y. (2001). Adaptation in vertebrate photoreceptors. Physiological Reviews, 80, 117-151.
Perlman, I., & Normann, R. A. (1998). Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Progress in Retinal and Eye Research, 17, 523-563.
Pugh, E. N., Jr., & Lamb, T. D. (2000). Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In D. G. Stavenga, W. J. de Grip & E. N. Pugh (Eds.), Handbook of biological physics, Vol. 3, Molecular mechanisms of visual transduction (pp. 183-255). Amsterdam: Elsevier.
Pugh, E. N., Jr., Nikonov, S., & Lamb, T. D. (1999). Molecular mechanisms of vertebrate photoreceptor light adaptation. Current Opinion in Neurobiology, 9, 410-418.
Stockman, A., Smithson, H. E., Michaelides, M., Moore, A. T., Webster, A. R., & Sharpe, L. T. (2007). Residual cone vision without α-transducin. Journal of Vision, 7(4), 8, 1-14.
Stockman, A., Smithson, H. E., Webster, A. R., Holder, G. E., Rana, N. A., Ripamonti, C., & Sharpe, L. T. (2008). The loss of the PDE6 deactivating enzyme, RGS9, results in precocious light adaptation at low light levels. Journal of Vision, 8(1), 10, 1-10.